More On: FDA
Hey, FDA! Let the birth control pill out of jail!
FDA Gives OTC Naloxone Pitch Another Go
Big Pharma says that drug prices reflect the cost of research and development
The FDA warns against the 'NyQuil Chicken Challenge,' which could be deadly
Before human testing is done, the latest COVID booster shots will be made available
The FDA should reclassify the opioid overdose antidote naloxone, approved for use in 1971 to rescue overdose victims for the past few decades, as an over‐the‐counter drug.
Also, all 50 states have found ways around the fact that the drug is only available with a prescription. Either they let state health directors and other licensed practitioners give standing orders to pharmacists to give the medicine to customers who ask for it, or they let pharmacists prescribe the drug. So, there is a lot of proof that people who are not doctors can and do use naloxone safely and correctly. Since more than 100,000 people die from overdoses every year, I can't say enough about how important it is to make this drug widely available.
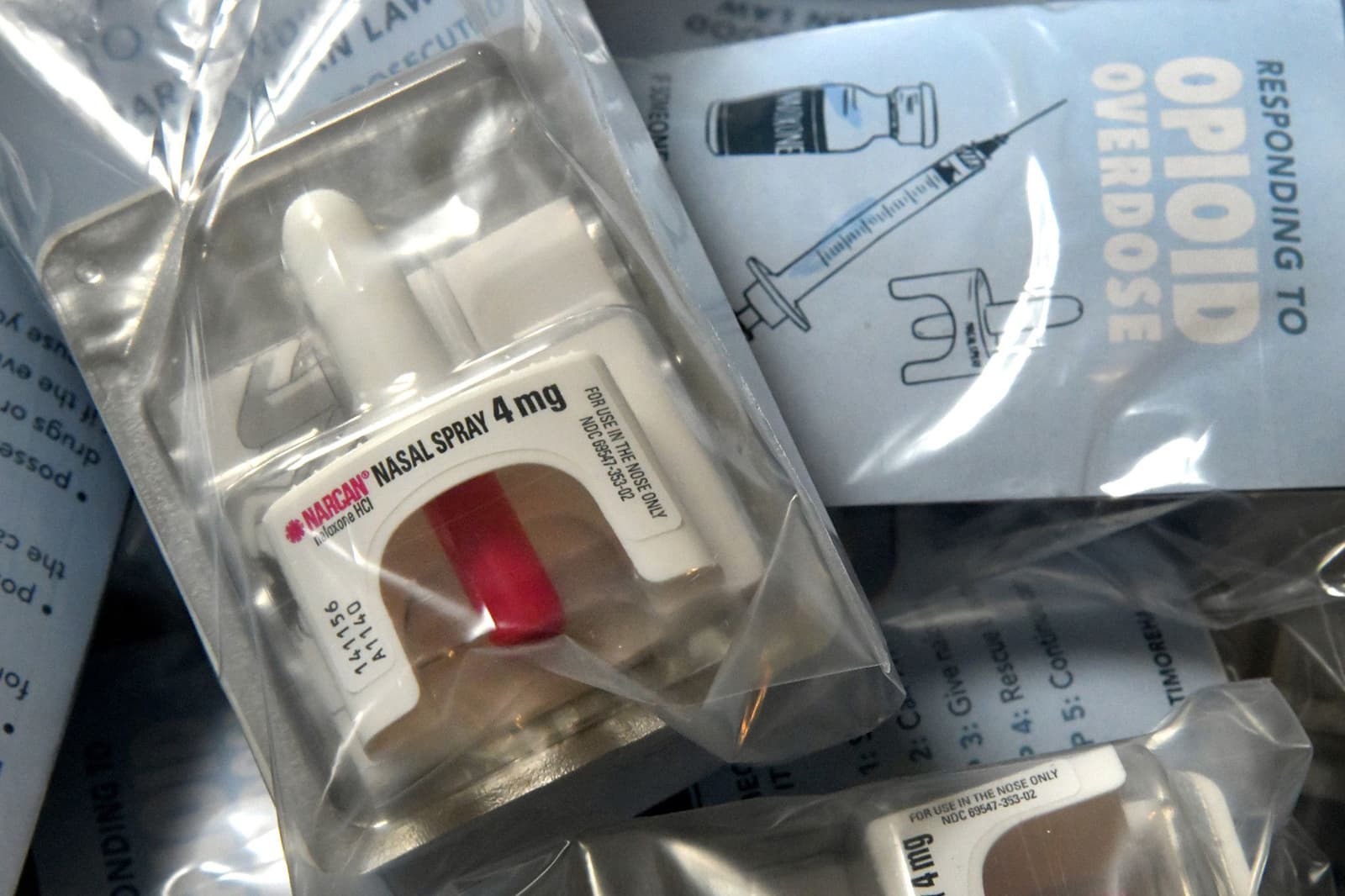
The FDA has told companies that make naloxone to ask the agency to start reclassifying it as an over-the-counter drug. In 2019, FDA Commissioner Scott Gottlieb went so far as to have the agency design the Drug Facts Labels (DFL) needed for drug companies to sell their products over the counter. These labels were then tested for "consumer comprehension" in front of focus groups, which is usually a job that drug companies have to do if they want their drugs to be reclassified as OTC. In the announcement, the Commissioner said that this is a first-of-its-kind effort to help and speed up the change from prescription-only to over-the-counter use of naloxone.
So far, nothing.
In a notice published today in the Federal Register, the FDA said that based on its "preliminary assessment," "certain naloxone products may be safe and effective for over-the-counter use." A press release from the agency said:
The Federal Register notice includes a preliminary assessment that certain naloxone drug products–up to 4 milligrams (mg) nasal spray and up to 2 mg autoinjector for intramuscular (IM) or subcutaneous (SC) use–may be approvable as safe and effective for nonprescription use. This preliminary assessment is intended to facilitate development and approval of nonprescription naloxone products; however, it is not a final determination that certain naloxone drug products are safe and effective for nonprescription use, and it does not mandate an immediately effective switch to nonprescription/over‐the‐counter (OTC) availability for naloxone.
As I have pointed out previously, the FDA doesn’t need to wait for the drug makers to request reclassifying a drug as OTC. Under FDA regulations, the FDA can undertake a reclassification review at the request of “any interested person” or the Commissioner himself. States may petition the FDA for reclassification. Finally, if all else fails, Congress can order the reclassification.
As Michael F. Cannon and I point out in our white paper, Drug Reformation:
Manufacturers often have little incentive to submit or support OTC petitions and every reason to oppose them, because removing prescription requirements for their or their competitors’ products brings greater price competition and reduced profits. The FDA’s policy of waiting for manufacturers to initiate prescription‐to‐OTC switches therefore rigs the process against consumers. If the power to require prescriptions remains with the FDA, the agency should initiate switches itself and abandon its historical practice of waiting for the manufacturers to petition the agency to switch their products.
The FDA’s Federal Register notice today is just another ill‐fated attempt to prod naloxone manufacturers into asking the agency to start the OTC reclassification process. Congress should respond to FDA fecklessness by imposing a deadline for the agency to make naloxone OTC.




















