More On: Coronavirus
Joe Biden Tests Positive for Wuhan Coronavirus Again
Aaron Rodgers, Green Bay Packers Fined for Violating COVID-19 Protocols
Shailene Woodley Defends Aaron Amid Rumored COVID-19 Quarantine Outings
Howard Stern: Aaron Rodgers Should Be Fired for 'Bulls--t' Vaccine Comments
Emilio Estevez Denies Being Anti-Vax After Exiting 'Mighty Ducks' Series
The coronavirus vaccine developed by Pfizer and BioNTech is more than 90 percent effective, the companies said Monday in a major milestone for the fight against the deadly global pandemic. The
The coronavirus vaccine developed by Pfizer and BioNTech is more than 90 percent effective, the companies said Monday in a major milestone for the fight against the deadly global pandemic.
The Manhattan-based drugmaker and the German biotech firm became the first pharmaceutical outfits to reveal successful data from a large, late-stage study of a COVID-19 vaccine at a time when the US and other nations are grappling with surging infection counts.
“Today is a great day for science and humanity,” Pfizer CEO Albert Bourla said in a statement. “With today’s news, we are a significant step closer to providing people around the world with a much-needed breakthrough to help bring an end to this global health crisis.”
Pfizer and BioNTech released the data from their crucial Phase 3 study of the vaccine after 94 of the trial’s 43,538 participants contracted COVID-19. An analysis showed the vaccine was more than 90 percent effective seven days after patients received the second of two doses, meaning they become protected from the virus 28 days after starting the vaccination course, according to the companies.
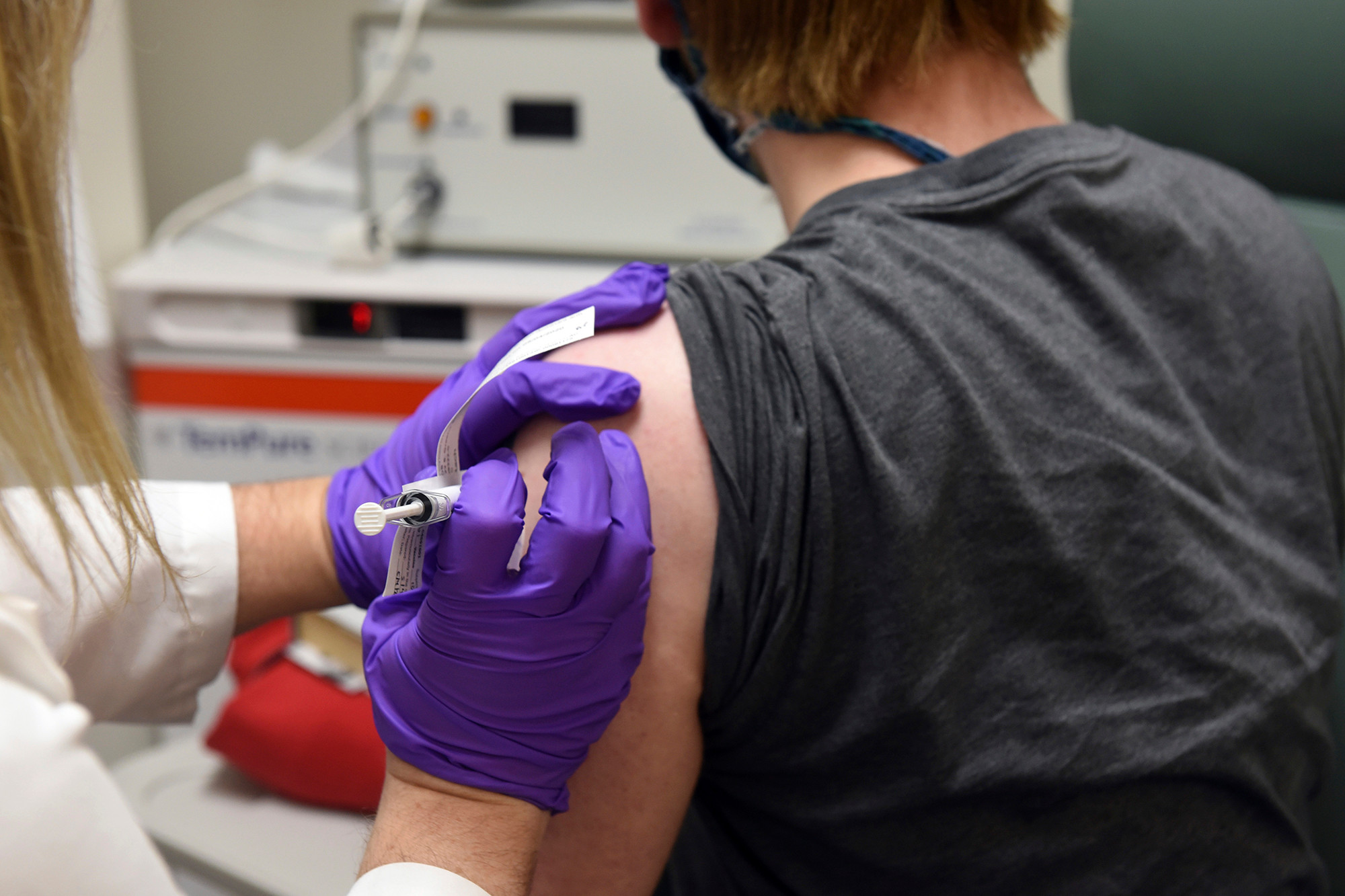
Pfizer and BioNTech will assess the vaccine’s effectiveness again when 164 study participants get sick with coronavirus. While the final efficacy rate will vary as the study continues, an independent committee tasked with examining the data has not reported any safety concerns, the companies said.
That rate is well above the 50 percent effectiveness that the Food and Drug Administration requires for a coronavirus vaccine and far higher than the typical efficacy of the seasonal flu shot, which was only 29 percent effective in the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.
The results suggest Pfizer’s shot works almost as well as the measles vaccine, which is about 93 percent effective with one dose and roughly 97 percent effective with two doses, the CDC says.
Monday’s results bode well for Pfizer’s plans to ask the US Food and Drug Administration to approve the vaccine for emergency use by the end of this month. The company will still have to prove the vaccine is safe, a milestone it expects to reach in the third week of November.
The news sent Pfizer’s stock price soaring about 14.3 percent in premarket trading to $41.61 as of 7:28 a.m.
Pfizer and BioNTech are just two of several major pharmaceutical firms that have been racing to develop a safe and effective COVID-19 inoculation since the start of the pandemic.
Three other potential vaccines besides Pfizer’s are currently undergoing large Phase 3 studies in the US, including candidates from Moderna, AstraZeneca and Johnson & Johnson.
With Post wires






















